specific heat metals box chem Specific Heat (cs): The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00 C. Specific heat is temperature (and phase) dependent. Thus, .
It could be done, you'd have to mount a pin in a mount clamped to the bottom of the quill which would duplicate the tool, 1/2 dia and as long as you 1/2" end mill. I could/can be done, but I've never done it, but then I don't inlet stocks.
0 · specific heats in metals
1 · specific heating properties
2 · specific heat of metals pdf
3 · specific heat of a substance
4 · specific heat capacity of metal
5 · metal specific heat formula
6 · metal specific heat calculator
7 · low specific heat capacity metals
A usinagem CNC, ou usinagem de controle numérico computadorizado, é um processo de fabricação moderno que utiliza máquinas automatizadas e programação de computador para criar peças e componentes precisos e complexos.
Specific heat of commonly used metals like aluminum, iron, mercury and many more - imperial and SI units. The specific heat of metals and metalloids (semimetals) are given in the table below. Specific heat online unit converter. See also tabulated values for gases, food . Specific Heat Capacity of Metals Table Chart. The specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree Celsius. The .Learning Objectives. Define heat capacity and specific heat capacity and differentiate between the two terms. Deduce which substance will have greatest temperature changed based on specific heat capacities. Calculate unknown . The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled. The equation that .
The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1 degree Celsius (or 1 kelvin):Specific Heat (cs): The quantity of heat liberated or absorbed when the temperature of 1.00 gram of a substance falls or rises 1.00 C. Specific heat is temperature (and phase) dependent. Thus, .
Heat capacity is a property that describes how much energy is needed to change the temperature of a material. Objects with a high specific heat capacity require a greater change in energy to .Specific Heat Capacity (C or S ) - The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity of the substance. The .
how to locate your leachfield distribution box
Specific heat online unit converter. See also tabulated values for gases, food and foodstuff, metals and semimetals, common liquids and fluids and common solids, as well as .
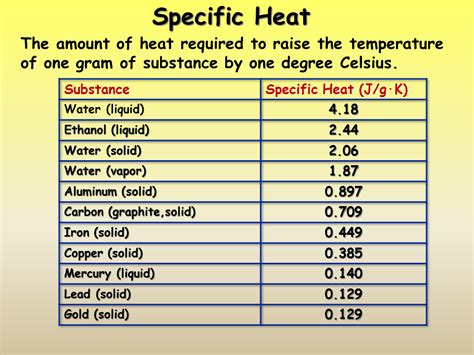
The specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the substance by \(1^\text{o} \text{C}\). The table below lists the .Question: Chem 65- Heat Capacity (Or Specific Heat) of Metals Objective: To identify an unknown metal by finding its specific heat. Procedure: 1. Mass an empty calorimeter (Styrofoam cup). 2. Fill the cup with about 100 ml of DI . Study with Quizlet and memorize flashcards containing terms like Thermodynamics, what is heat, Specific heat (C) and more. . Specific Heat of Metals Lab. Flashcards; Learn; Test; Match; Q-Chat; Flashcards; Learn; Test; Match; . Organic Chemistry Chapter 11 (Spectroscopy) 34 terms. mbailey_24. Preview. Unit 7 Study Set - Kramer. 10 .
An electrode consists of an electronic conductor in contact with an ionic conductor according to the definition accepted in electrochemistry. 1 Two electrodes, called sometimes half cells, make a galvanic cell. The potentials of .
Chemistry questions and answers; LAB: Heat Effects & Calorimetry Determining the Specific Heat of a Metal Introduction Heat is a form of energy, sometimes called thermal energy, which can pass spontaneously from an object at a high temperature to an object at a lower temperature. . The specific heat of a metal is related in a simply way . Chemistry 107 York College/CUNY Lab #4 Online Summer 2020 Chemistry 107: ONLINE Experiment 4 Specific heat of a Metal INTRODUCTION In this experiment you will use calorimetry to determine the specific heat of a metal. When a substance is heated, the motion of its individual particles increases, resulting in an increase in temperature. The more heat added . The heat capacity of an object depends both on its mass and its chemical composition. Because of its much larger mass, the swimming pool of water has a larger heat capacity than the wading pool. . Water is very resistant to changes in temperature, while metals generally are not. The specific heat of a substance is the amount of energy .
Chemists identify substances on the basis of their chemical and physical properties. One physical property of a substance is the amount of energy it will absorb per unit of . The specific heat of the metal can now be calculated: Specific heat, C = heat .4. Results and Discussion The following table shows the masses of the objects used, their specific heat capacity, and the initial and final temperature to determine the heat of given up by the calorimeter . This will then determine the specific heat capacity of the metal. Table 1. Specific heat capacity of the metal Image 13.The specific heat of water is 4.184 J/g C. Thus, the heat gained by the water can be calculated. From the zero’th law the amount of heat must be equal to the heat lost by the metal sample. From this, the specific heat of the metal is calculated.
(Solved): chem21 specific heat capacities of metals . chem21 specific heat capacities of metals. We have an Answer from Expert View Expert Answer. Expert Answer . . Chemical Engineering Electronics and Communication Engineering Mathematics Physics Chemistry Software Works/ Computer Science Other Subjects.
B. Calculate values for the specific heats for the two unknown metals. For Unknown I the heat gained by water : qhot metal = - qcold water (mass * Cs * ∆T)hot metal = - (50.0 g * 4.184 J g ˚C * (35.03 ˚C – 20.0 ˚C))cold water so the metal must have lost that much heat and the specific heat would be (mass * Cs * ∆T)hot metal = –1.35 x .Chemistry; Chemistry questions and answers; EXPERIMENT 1: DETERMINATION OF SPECIFIC HEAT OF A METAL Data Sheet Table 2: Mass Mass (g) Water 50.Aglin cup) cup is 2.8, 11.89 Unknown Metal Strip Table 3: Specific Heat Data Time (minutes) Temperature (°C) Trial 2 Trial 3 Trial 1 Initial 5 minutes 24 °C 6 minutes 24°C 7 minutes 24°C 72 | 23°C .
How to Use Specific Heat Capacity to Calculate Heat Energy Transfer. Specific heat capacity, often simply called specific heat, is a property of a substance that describes how much heat energy it requires to raise the temperature of one kilogram of the substance by one degree Celsius (or one Kelvin). Different substances have different specific heat capacities, which .16.1_reinforcement notes Chemistry For 101; Specific heat capacity problems; Science Class Science Claas; Related documents. LAB Journal - penny lab journel; Tsunami+Menu+Update+6; 5ada8df6 d038 488e 8a38 499513434 db9; Dropbox - school work; Thinkbox - school work; honors chem 8.03 lab; English (US) United States.
Table 2: Literature Values of Specific Heat Capacity Metal Specific heat capacity (J g℃) Density (g cm3) Metal Specific heat capacity (J g℃) Density (g cm3) Gold 0.13 19.3 Zinc 0.39 7.14 Lead 0.13 11.4 Iron 0.45 7.86 Silver 0.24 10.49 Aluminum 0.90 2.70 Copper 0.39 8.96 Magnesium 1.02 7.44 9.4.5 Calculate Percent Difference link-http://www.kentchemistry.com/links/Energy/SpecificHeat.htmThis advanced video takes you through the procedure for solving for the final .
specific heats in metals
classify matter & specific heat chem test. Flashcards. Learn. Test. Match. Representative metals. Click the card to flip 👆. Exhibit well known physical and chemical properties they are group A metals.Study with Quizlet and memorize flashcards containing terms like Beryllium is a rare metal that is gray in color, strong, and lightweight. The following data was obtained from a calorimeter experiment: The specific heat of beryllium is _____ J/g°C 0.124 0.549 1.82 9.61 9520, The lab procedure involves several factors, listed below. Some were variable and some were constant. Specific heat capacity is used with the mass of a substance to determine the q of a reaction. Molar heat capacity is used with moles, and heat capacity is the amount of heat required to raise the temperature of an object by 1 degree Celsius. To calculate the specific/molar heat capacity, you divide the heat capacity by the mass or moles of . EISCO 6pc Specific Heat Metal Cylinders Set - Copper, Lead, Brass, Zinc, Iron & Aluminum . 999 Pure Chemistry Element Design with Certificate of Authenticity by Unique Metals. . Box Office Mojo Find Movie Box Office Data: Goodreads Book reviews & recommendations : IMDb Movies, TV
how to lay sheet metal roofing
In the Specific Heat: Chemistry Demonstration—ChemTopic™ Lab Activity, three different metals of equal mass are heated to the same temperature then added to three insulated foam cups with equal amounts of water. Explore why the resulting temperature changes are different for the three metals. Available as part of the Thermochemistry—ChemTopic™ Labs digital collection.
Chemistry; Chemistry questions and answers; 1. The specific heat capacity of a metal can be determined by heating it in a water bath, pouring the hot metal into cool water, and measuring the change in temperature. List two assumptions that are .[AP Chem: Specific Heat Capacity] Chemistry This is summer work so I haven’t learned it yet but I’m unsure where I’m going wrong. I’m not exactly sure what I’m supposed to be doing either.help would be greatly appreciated! . This number also makes sense because metals have low specific heat capacity (i.e. easy to heat up) Reply reply You are certain that the metal is either copper (specific heat=0.093 cal.g C), lead (specific heat=0.031 cal/g), or aluminum (specific heat=0.22 cal/g C). You run an experiment in which you find that the metal absorbs 6.2 calories of heat when it .
specific heating properties
(Hint: the heat lost by the water equals the heat gained by the ethanol. Assume no heat loss to the surroundings). Show all work to receive full credit. Put a box around your final answer. Metal Specific Heat (J/g C) Steel 0.46 Lead 0.130 0.903 Brass .
Study with Quizlet and memorize flashcards containing terms like how much energy is needed to raise the temperature of 10g of iron compared to 10 g of aluminum each by 1° c, 25.0 g of each material has 150 j of energy added which material has the largest increase in temperature, 10 g samples of three of different metals each initially at 20° c are left in the Sun for 10 minutes the .
Maximum temperature of metal and water from graph (Celsius) 26. Temperature change of water, T (Celcius) 3. Calculations for specific heat of a metal; Heat gained by water (J) 317. Temperature change of metal, T (Celcius) -70. Specific heat of metal, (J/gC) 0. Trial 1 Trial (NH 4 Cl) Mass of salt (g) 1 1. Moles of salt (mol) 0 0.
specific heat of metals pdf
New and used Storage Shelves for sale near you on Facebook Marketplace. Find great deals or sell your items for free.
specific heat metals box chem|metal specific heat calculator